Overview
We are developing unique analytical methods for studying the tumor microenvironment to comprehensively analyze interactions between the immune system and cancer cells. By establishing the concept “immunogenomic cancer evolution”, which holds that genetic abnormalities in cancer cells directly affect immune responses, we are pioneering a new field in tumor biology. Since the immune system is affected by genetic and environmental diversity, we explore elements involved in immune response analyzing comprehensive data from cancer patients and verify the universal significance of each element in a mouse model.
Members
Professors

Hiroyoshi Nishikawa





Research Focus
The clinical application of cancer immunotherapy has demonstrated that “resurgence” of immune surveillance is achievable in humans. However, it has also been uncovered that the resurgence of immune surveillance is affected by various environmental factors, such as energy and substance metabolism, as well as genetic diversity on both the cancer and host side. In our research, we elucidate the regulatory mechanisms of immune tolerance and surveillance by integrating immunology, genomic medicine, metabolism, and bioinformatics. We investigate molecular expression and spatiotemporal dynamic changes of individual immune cells at the single-cell level and link alterations in intercellular networks to macrobiological perspectives at the tissue and individual levels. To achieve this, we conduct integrated research using genetically-engineered mice that allow us to track and examine each immune cell population based on findings from cancer patients. Furthermore, through the AMED Moonshot Project, we elucidate the dynamics of immune responses during the process where normal tissue cells acquire somatic mutations and become “cancerous” due to chronic inflammation. This project also aims to expand our understanding of preventive medicine.
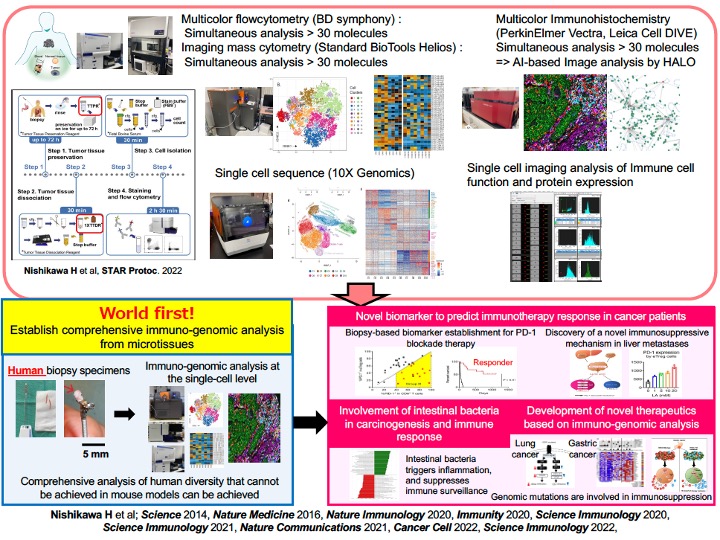
The system and research equipment for conducting research of cancer cells and immune cells at the single-cell level (immune-genomic analysis). Also, an introduction of some of the research results to date.
Selected Publications
Kumagai S, Itahashi K, Nishikawa H: Regulatory T cell-mediated immunosuppression orchestrated by cancer: towards an immuno-genomic paradigm for precision medicine. Nat Rev Clin Oncol. 21(5):337-353. 2024. DOI: 10.1038/s41571-024-00870-6
Itahashi K, IrieT, Yuda J, Kumagai S, Tanegashima T, Lin Y-Z, Watanabe S, Goto Y, Suzuki J, Aokage K, Tsuboi M, Minami Y, Ishii G, Ohe Y, Ise W, Kurosaki T, Suzuki Y, Koyama S, Nishikawa H: BATF epigenetically and transcriptionally controls the activation program of regulatory T cells in human tumors.Sci Immunol 14;7(76):eabk0957 2022.
Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin, Y-T, Togashi Y, Kamada T, Irie T, Okumura G, Kohno H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakaumra H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Cida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H: Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell.40(2):201-218.e9 2022. DOI: https://doi.org/10.1016/j.ccell.2022.01.001
Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Kochin V, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H: The PD-1expression balance between effector and regulatory T cell predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol.21(11):1346-1358 2020.
Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H: An oncogenic alteration creates a tumor microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. 53(1):187-203.e8 2020. DOI: 10.1016/j.immuni.2020.06.016
Recruitment & Contact
We are permanently recruiting highly motivated students and postdoctoral fellows, regardless of previous scientific backgrounds.
Please contact us using CCII’s contact form or by directly sending an email to recruit_ccii@mail2.adm.kyoto-u.ac.jp. Please indicate in which position and subject area you are interested in and that your inquiry is about our research division.
